
Bromine
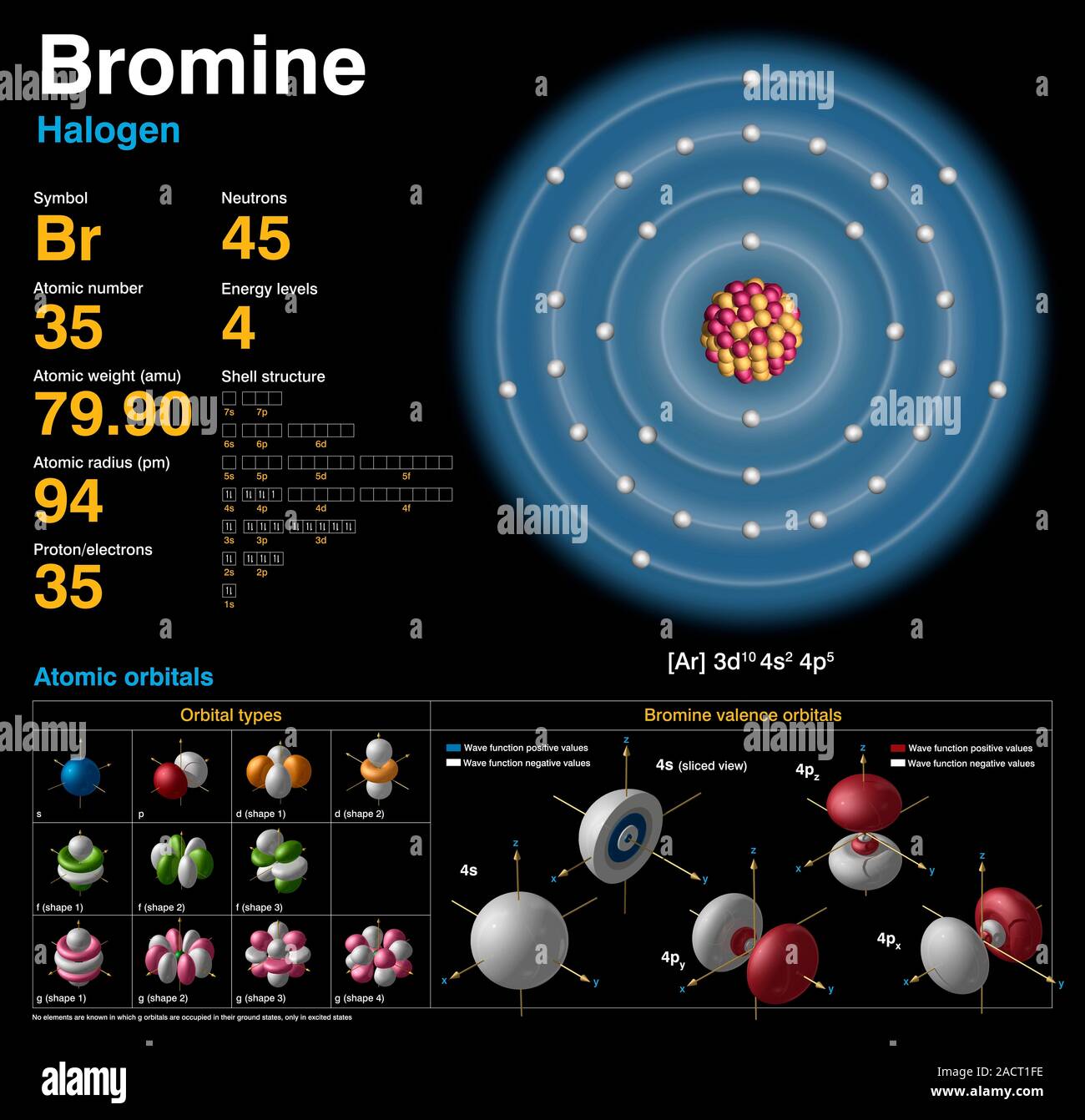
Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. The chemical symbol for Bromine is Br. Bromine is the third-lightest halogen, and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas. Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Atomic number of bromine is 35, its electronic configuration is 2, 8, 18, 7. The detailed configuration is Br: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5.
| Atomic Number: | 35 | Atomic Radius: | 183 pm (Van der Waals) |
| Atomic Symbol: | Br | Melting Point: | -7.2 °C |
| Atomic Weight: | 79.90 | Boiling Point: | 58.8 °C |
| Electron Configuration: | [Ar]4s23d104p5 | Oxidation States: | , 5, 4, 3, 1, −1 (a strongly acidic oxide) |
History
From the Greek word bromos, Daniel craig new movie knives outlet. stench. Discovered by Balard in 1826, but not prepared in quantity until 1860.

Sources
A member of the halogen group, bromine is obtained from natural brines from wells in Michigan and Arkansas. Some bromine is extracted today from seawater, which contains only about 85 ppm.
Properties
Atomic Number Of Brass
Bromine is the only nonmetallic liquid element. Patrick stewart memoir. It is a heavy, mobile, reddish-brown liquid, volatilizing readily at room temperature to a red vapor with a strong disagreeable odor, resembling chlorine, and having a very irritating effect on the eyes and throat; it is readily soluble in water or carbon disulfide, forming a red solution, is less active than chlorine but more so than iodine; it unites readily with many elements and has a bleaching action; when spilled on the skin it produces painful sores. It presents a serious health hazard, and maximum safety precautions should be taken when handling it.
Production
Much of the bromine output in the U.S. was used in the production of ethylene dibromide, a lead scavenger used in making gasoline anti-knock compounds. Lead in gasoline, however, has been drastically reduced due to environmental considerations. This will greatly affect future production of bromine.
Atomic Number Of Boron 10
Uses
Br Atomic Number 35
Bromine is used in making fumigants, flameproofing agents, water purification compounds, dyes, medicines, sanitizers, inorganic bromides for photography, etc. Organic bromides are also important. Android apps.com.
